|
Real
Benefits with 3gAllergy™
Run
both routine immunoassay and allergy testing at the same time. With a
menu of about 350 allergens and allergy panels.
The future is now:
DPC's 3gAllergy, the only third-generation allergy assay
Finding the right solution for allergy testing has been a challenge for
many—until now. DPC's 3gAllergy™, the only FDA-cleared third-generation
allergen-specific IgE assay, is a revolutionary in vitro blood test that
has brought laboratories everything they have been waiting for: speed,
convenience and quality performance, all in one assay. 3gAllergy stands
apart from all others, on the basis of its system attributes and assay
design. While its evolution has spanned three generations of technology,
3gAllergy has proved to be well worth the wait.
The first generation
Allergy testing began with the development of the radioallergosorbent
test (RAST), which resulted in the detection of allergen-specific IgND
antibodies. The presence of these antibodies in serum was concordant with
skin testing. The subsequent discovery of IgE prompted the development
of the first generation of IgE immunoassays. The acronym RAST has been
used as a generic term for in vitro allergy testing ever since.
RAST methodology offers
diagnostic values of serum allergen-specific IgE similar to those of the
skin prick test (SPT), but RAST demonstrates much higher reproducibility
and is the test of choice with patients suffering from severe urticaria
and eczema. Another noticeable clinical advantage of RAST concerns patients
prone to anaphylactic reactions with exposure to even minute amounts of
allergen. In addition, the diagnostic values of RAST are not affected
by pharmacotherapy administered for allergy symptoms. RAST became an established
laboratory tool to assist in allergy diagnostics on the basis of its clinical
features, dependable results, and the convenience it offers patients.
In Europe, allergy specialists were receptive to the first-generation
RAST methodology.
The second generation
The limitations of first-generation in vitro allergen-specific IgE assays
signaled the need for an improved second-generation assay that could be
run on an automated instrument. The criteria for development of a second-generation
in vitro allergy test focused on the following characteristics:
 |
 |
Conformance
to NCCLS guidelines for precision and functional sensitivity |
| |
 |
WHO
(World Health Organization) calibration standardization (75/502) |
| |
 |
Timely
incubation (time-to-first-result) |
| |
 |
Superior
detection signal |
| |
 |
Improved
detection and binding to antibody |
| |
 |
Improved
and uniform test results to report IgE classes. |
The advent of the
second-generation in vitro allergy test, also known as modified RAST (mRAST),
turned the second-generation in vitro allergy test into a mainstream assay.
On account of several unrelated issues, allergy specialists refrained
from fully endorsing RAST and mRAST in the US. The key determining factor
for taking this stance was that SPT was deemed to be more sensitive than
RAST and mRAST. Owing to the alarming growth of allergy in the US, however
(where approximately 50 million Americans suffer from allergy-related
symptoms), and since the cost for allergy was approaching $20 billion
per year, the American healthcare system favorably incorporated the first-
and second-generation assays into its formulary.
Still, the needs of
laboratories were not being adequately met. Extrinsic pressures on laboratories
mandated substantial improvements to first- and second-generation methodologies
for in vitro allergy testing. Economic forces dictated radical changes
to improve assay performance and to develop an instrument capable of justifying
its cost by addressing the day-to-day expectations of laboratory managers
and fulfilling the demands of business managers.
The
third generation
A manufacturing pioneer
and a worldwide leader in the development of immunoassays, Diagnostic
Products Corporation (DPC) was among the few companies that recognized
the diagnostic value of IgE in the detection of allergies. During the
last 20 years, DPC has dedicated considerable research and development
resources towards the latest generation in vitro allergen-specific IgE
testing methodology. DPC has successfully transitioned from first-generation
in vitro allergen-specific IgE assays to the only FDA-cleared third-generation
in vitro allergen-specific IgE assay, marketed under the name 3gAllergy.
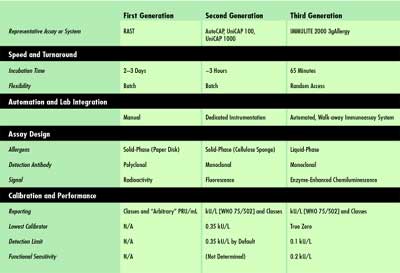
For a better understanding of the added attributes of first-, second-
and third-generation in vitro allergen-specific IgE assays, and how they
are differentiated, see Table 1.
|
Table
1. A generational classification
of allergen-specific IgE assays.
|
 Click image to
view full size.
Click image to
view full size. |
|
|
| 3gAllergy™
runs on the IMMULITE® 2000 and the IMMULITE®
2500 systems. Ask your DPC representative for more information. |
The prevalence of
allergic disease and the drastic changes in the US healthcare paradigm
require that primary care physicians, pediatricians and other medical
specialists manage and treat patients with allergies. The ongoing changes
in the healthcare system have created a stronger diagnostic role for laboratories.
Early on, DPC recognized
the global healthcare market's need for a new in vitro allergy assay and
an automated system. During the first DPC International Conference on
Allergy, held in June, 2003 in Paris, France, Michael Ziering, President
and CEO, announced DPC's commitment to dedicate appropriate resources
to allergy diagnostics over the coming years and to provide the most advanced
methodology to laboratories, along with the necessary educational programs
and support.
3gAllergy has enabled
DPC to meet the needs of laboratories of any size. From a simple serum
sample, laboratories are able to generate fast and accurate test results
that provide physicians with valuable clinical information. DPC's unique
liquid allergens contribute greatly to 3gAllergy's sensitivity, specificity
and reliability. The soluble polymer/copolymer support for the allergens
increases the accessibility of binding sites and their accessibility to
allergen-specific IgE antibodies. DPC's proprietary wash technique maximizes
the removal of excess reagent and ensures adequate separation of bound
from unbound materials.
The enzyme-enhanced
chemiluminescence technology allows for greater accuracy and precision
compared to other signal detection methods. A zero calibrator ensures
a true 0.1 kU/L detection limit. 3gAllergy's calibrators are standardized
against the WHO 2nd International Reference Preparation (IRP) 75/502.
Assay results are reported quantitatively as well as in standard and extended
class scores.
Today's solution
3gAllergy is the right solution for today's laboratories. It is now universally
accepted that the gold standard in allergy testing consists of either
of the following: 1) a patient clinical history + an in vitro test or
2) a patient clinical history + an in vivo test.
As the unique features
and benefits of DPC's third-generation methodology have fulfilled the
expectations of laboratories, it is essential to create awareness among
physicians. First- and second-generation in vitro allergy tests have demonstrated
their usefulness as laboratory diagnostic tests to physicians. Numerous
studies have concluded the clinical utility of in vitro allergen-specific
IgE tests. 3gAllergy can assist physicians in identifying allergens responsible
for evoking allergy symptoms, predicting future allergy development, and
guiding clinical management and treatment of allergy patients. Its functional
sensitivity of 0.2 kU/L allows for the detection of existing allergies
in infants and can help predict the future risk of developing allergy-induced
diseases such as asthma1. It also facilitates
timely therapeutic intervention. DPC is determined to cooperate on a worldwide
basis with key research centers and opinion leaders to evaluate the clinical
significance of third-generation criteria, and share its findings with
healthcare providers for the ultimate benefit of patients.
| References |
| 1. |
Sasai
K, Furukawa S, Muto T, Baba M, Yabuta K, Fukuwatari Y. Early detection
of specific IgE antibody against house dust mite in children at risk
of allergic disease. J Pediatr 1996;128:834-40. |
|